Answer:
Option B
Explanation:
$SO_{2}$ oxides can act both as an oxidising agent as well as a reducing agent. It behaves as a reducing agent in moist conditions. It converts iron (III) ions to iron (II) ions and decolourises acidified potassium permanganate(VII) solution.
$2 Fe^{3+}+ SO_{2}+2H_{2}O\rightarrow2Fe^{2+}+SO_{4}^{2-}+4H^{+}$
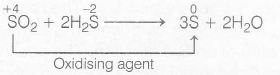
when SO2 reacts with H2S, it produces sulphur and water. In this reaction, 'SO2' act as an oxidising agent.