Answer:
Option A
Explanation:
Arenes on treatment with chlorine in presence of Lewis acid catalyst, ferric chloride or aluminium chloride and in the absence of light undergoes halogenation. It involves an electrophilic substitution reaction

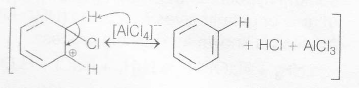
Mechanism of electrophilic substitution in as follows:
Step I: Generation of an electrophile.
$Cl-Cl+AlCl_{3}\rightarrow Cl^{+}+Cl ^{\delta+}.........Al^{\delta-}-Cl_{3}$
$\rightarrow Cl^{+}+[AlCl_{4}]^{-}$ Chloronium ion
Step II. Formation of carbocation (arenium ion)

The arenium ion gets stabilised by resonance

Step III removal of proton