Answer:
Option B
Explanation:
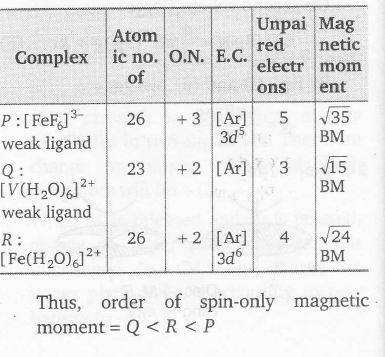
Plan spin only magnetic moment $=\sqrt{N(N+2)}$ BM, when N is the number of unpaired elecrons. In presence of weak ligand (as H2O, Cl-, F-) there is no pairing of electrons, and electrons donated by ligands are filled in outer vacant orbitals
In presence of strong ligand
(as CN-, CO, NH3,en) electrons are paired and electrons from ligands are filled in available inner orbitals