Answer:
Option D
Explanation:
Nitric acid not only nitrates but also oxidizes the highly reactive ring as well, with loss of much material as dark-coloured tar. Furthermore, in the strongly acidic nitration medium, the amine is converted into anilinium ion (-NH3+): substitution is thus controlled not by the -NH2 group but the -NH3 + group which because of its positive charge directs the entering group to the meta-position instead of ortho, and para
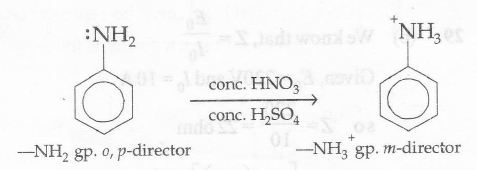
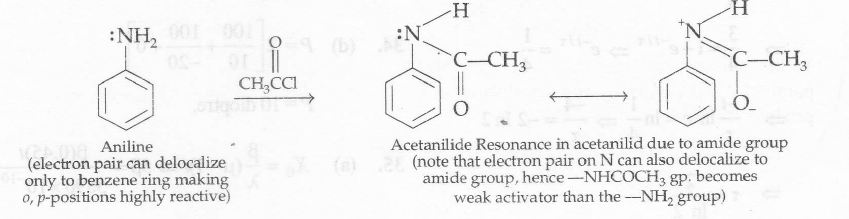
However, all these difficulties are overcome by protecting the amino group by acetylation, with either acetyl chloride or acetic anhydride. Acetylation (-NH2→ NHCOCH3) coverts -NH2 group to acetamido (-NHCOCH3) group which is 0, p-directing but lesser activating toward electrophilic aromatic substitution than the parent -NH2 group.