Answer:
Option B
Explanation:
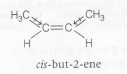
Boiling point =$37^{0}C(\approx$277k)
$\mu=0.33D$
cis-but-2ene has higher dipole-dipole attraction than trans-isomer hence, has high boiling point.
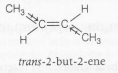
Boiling point=0.9= ° C( $\approx$ 274 K)
$\mu=0D$
the dipole moment of trans-but-2ene is zero because the bond dipoles cancel each other.
However, the dipole moment of cis-but-2-ene is 0.33 D , the vector sum of the two bond dipoles