Answer:
Option B
Explanation:
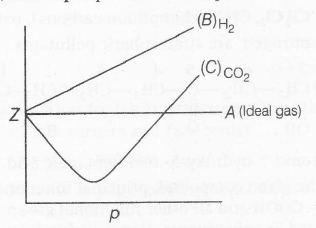
The extent of deviation shown by real gases from ideal behaviour is measured in terms of compressibility factor (Z) which is defined as the ratio of pV and nRT.
Mathematically,
Z=pVnRT
for ideal gases, Z=1
Easily liquefiable gases such as CO2 exhibit larger deviation as compared to gases such as H2,O2,N2 etc., that liquefy with difficulty.