Answer:
Option C
Explanation:
In ferrimagnetic substances, the magnetic moment of the domains are aligned in parallel and antiparallel directions in unequal number. The net magnetic moment is small. Thus, these are weakly attracted by magnetic field than ferromagnetic substances and these also lose ferrimagnetism on heating and become paramagnetic. e.g. $Fe_{3}O_{4}$ and ferrite like $ZnFe_{2}O_{4}, MgFe_{2}O_{4}$ and $Ni Fe_{2}O_{4}$ etc
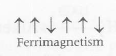
Among the given options , we have 3 compounds (i.e, $Fe_{3}O_{4},MgFe_{2}O_{4}$ and $NiFe_{2}O_{4}$) that show ferrimagnetism.
Hence, option (c) is the correct answer