Answer:
Option A
Explanation:
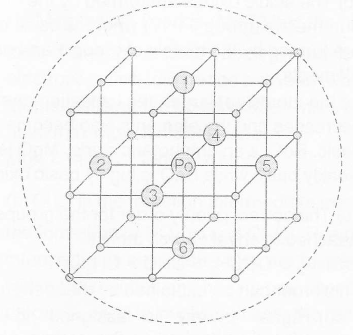
In a simple cubic structure, the spheres are not packed as closely as they could be, and they only fill about 52% of the volume of the container. This is a relatively inefficient arrangement and only one metal (polonium, Po) crystallises in a simple cubic structure. For a polonium atom in a simple cubic array, the coordination number is six.