Answer:
Option C
Explanation:
Key Idea Lewis acids are defined as, "Electron deficient compounds which have the ability to accept at least one lone pair,"
The compound given are
PH3- Octet complete although P has vacant 3d-orbital but does not have the tendency to accept lone pair in it. Hence, it cannot be considered as Lewis acid.
BCl3- In complete octet with following orbital picture.
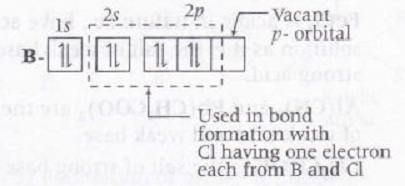
Hence, vacant p-orbital of B can accept one lone pair thus it can be considered as Lewis acid.
AlCl3 - Similar condition is visible in AlCl3 as well i.e.
Al( Valance orbital only)=
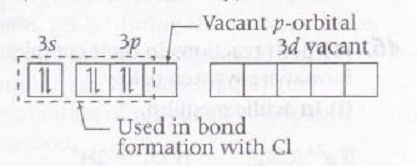
Hence this compound can also be considered as Lewis acid.
SiCl4 - Although this compound does not have incomplete octet but it shows the tendency to accept lone pair of electrons in its vacant d- orbital. This tendency of SiCl4 is visible in the following reaction.
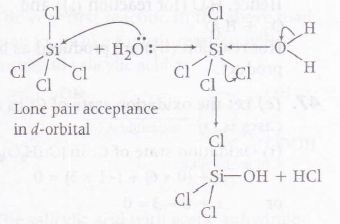
Thus option (b) and (c) both appear as correct but most suitable answers is (c) as a condition of a proper Lewis acid is more well defined in BCl3 and AlCl3.