Answer:
Option B,C
Explanation:
If there is inversion of specific rotation from (+) to (-), then invert sugar is formed.
(a) $C_{12}H_{22}O_{11}[(+)maltose140^{0}]+H_{2}O\rightarrow Glucose [D(+) 52^{0}]$
(b) $ C_{12}H_{22}O_{11}[(+)sucrose+66^{0}]+H_{2}O$
$\rightarrow Glucose [D(+) 52^{0}]+Fructose [L(-)-92^{0}] $
-40° for 2 moles mixture
-20° for 1 mole mixture
There is formation of invert sugar, Thus, correct
(c) Specific rotation of invert sugar is -20° per mole. Thus, correct
(d) $Br_{2} $ water is a weak oxidising agent.
It oxidises -CHO to -COOH.
$- CH_{2} OH$ group is not affected
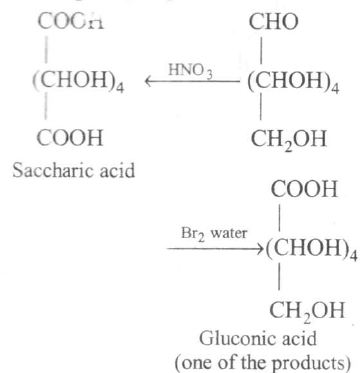
$HNO_{3}$ (a strong oxidising agent) oxidises invert sugar to saccharic acid. Thus, incorrect