Answer:
Option B,C,D
Explanation:
(a) Nearest neighbour in the topmost layer of ccp structure is 9 thus, incorrect
(b) Packing efficiency is 74% thus, correct
(c) Tetrahedral voids=2
Octahedral voids= 1 per atom thus, correct.
(d) Edge length,
a=4√2r=2√2r
thus, correct explanation
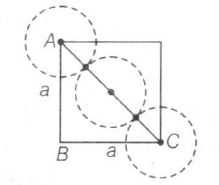
Edge length=a, Radius=r
AC2=AB2+BC2
(4r)2=a2+a2=2a2
4r=√2a⇒r=√24a=a2√2
∴a=2√2r
In ccp structure, the number of sphere is 4,
Hence , volume of 4 spheres= 4(43πr3)
Total volume of unit cell= a3=(2√2r)3
% of packing efficiency= volume.of4spheresvolume.ofunitcell
= 4(43πr3)(2(√2r))3×100=74.05%= 74%