Answer:
Option D
Explanation:
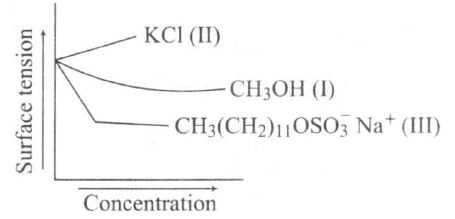
$ I. (CH_{3}OH)$: Surface tension
decreases as concentration increases.
II. (KCI): Surface tension increases with concentration for ionic salt.
III. $[CH_{3}(CH_{2})_{11}OSO_{3}^{-}Na^{+}].$: It is an anionic detergent.
There is a decrease in surface tension before micelle formation. and after CMC (Critical Micelle Concentration) is attained. no change in surface tension.