Answer:
Option A
Explanation:
- OH group displays both kinds of effect:
an electron-withdrawing acid - strengthening inductive effect from the meta-position and an electron releasing acid weakening resonance effect from the para-position (at this position. resonance effect overweighs the inductive effect). Thus, III>IV
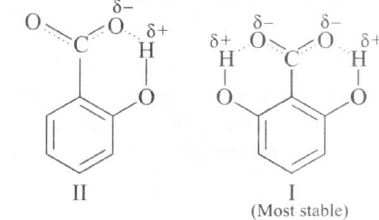
o- hydroxybenzoic acid (II) is far stronger than the corresponding meta and para isomers as the carboxylate ion is stabilised by intramolecular H-bonding
2.6-dihydroxybenzoic acid (I) forms carboxylate ion which is further stabilised by intramolecular H-bonding. Thus correct order is I>II>III>IV