Answer:
Option C
Explanation:
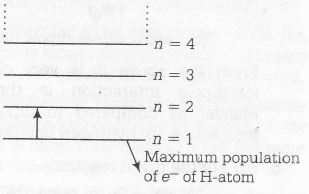
Since, at n=1, the population of electrons.
is maximum i.e, at ground state, So maximum
excitation will take place from n=1 to n=2
Hence, n=2 is the possible excited state.
Now, we have the formula for energy of H-atom
$\left(E_{n}\right)_{H} =-13.6 \frac{Z^{2}}{n^{2}}eV$
where, Z= atomic number, z for H-atom- 1.
$\therefore$ $\left(E_{n}\right)_{H} =-13.6\times \frac{{1}}{2^{2}}eV$
= -3.4 eV