Answer:
Option A
Explanation:
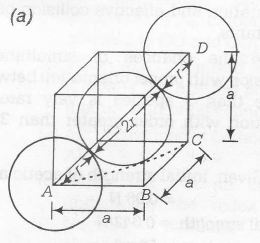
From this figure
$(AC)^{2}=(AB)^{2}+(BC)^{2}$
$(AC)^{2}=a^{2}+a^{2}=2a^{2}$
Also, $(AD)^{2}=(AC)^{2}+(DC)^{2}$
$(4r)^{2}=(2a)^{2}+a^{2}$
$16r^{2}=3a^{2}$
$r=\frac{\sqrt{3}}{4}a$
Now, when Na metal crystallise in bcc unit cell with unit cell edge, a= 4.29Å
We have the formula for radius
i.e, $r=\frac{\sqrt{3}}{4}\times4.29$ Å =1.86Å