Answer:
Option B
Explanation:
Plan This problem is based on concept of quantum number. Follow the following steps to solve this problem.
Write all possible orbitals having combination of same principle, azimuthal magnetic and spin quantum number.
Then count the all possible electrons having given set of quantum numbers
For n=4, the total number of possible orbitals are
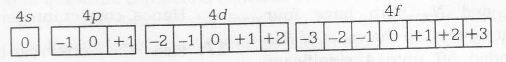
According to question |m1|=1, i.e, there are two possible values of m1, i.e, +1 and -1
and one orbital can contain maximum two electrons one having s=+1/2 and other having s=-1/2
So, total number of orbitals having (|m1|=1)=6
Total number of electrons having (|m1|=1 and m2=-1/2) =6