Answer:
Option B,D
Explanation:
(a) It does not undergo self ionisation in water but accepts an electron pair from water so it behaves as a weak monobasic acid
$H_{3}BO_{3}+H_{2}O\rightleftharpoons B(OH)_{4}^{-}+H^{+}$
Hence, (a) is incorrect.
(b) When treated with 1,2 -dihydroxy or polyhydroxy compounds, they form chelate (ring complex) with effectively remove [B(OH)4]- species from solution and thereby produce the maximum number of H3O+ or H+ ions. i,e, results in increased acidity.
(c) Baric acid crystallises in a layer structure in which planar triangular BO33- ions are bonded together through hydrogen bonds.
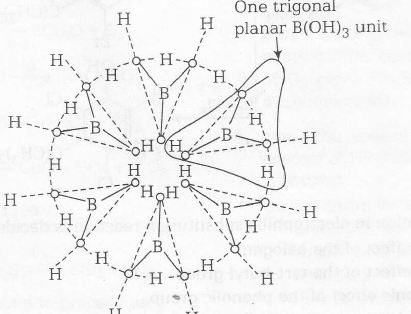
(d) In water the pKa value of H3BO3 is 9.25
$H_{3}BO_{3}+H_{2}O\rightleftharpoons B(OH)_{4}^{-}+H^{+}:pK_{a}=9.25$
So, it is weak electrolyte in water