Answer:
Option A,B,C
Explanation:
Plan This problem includes concept of effect of steric and electronic effect on reactivity of organic compounds.
Steric effect of halogens are as follows Cl2 < Br2 < I2
The electronic effect of phenolic group directs the approaching electrophile towards ortho and para positions. Tertiary butyl group has large size so it causes steric effect around aromatic nucleus. On the basis of the above factors the products of the given reactions are as follows
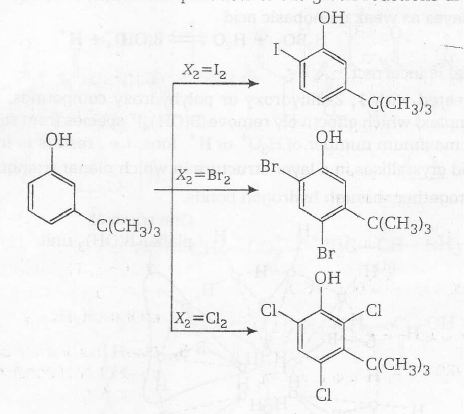
Hence, orientation in electrophilic substitution reaction is decided by
(a) The steric effect of the halogen
(b) the steric effect of the tert-butyl group
(c) the electronic effect of the phenolic group
(a),(b),and (c) are correct choices.