Answer:
Option B
Explanation:
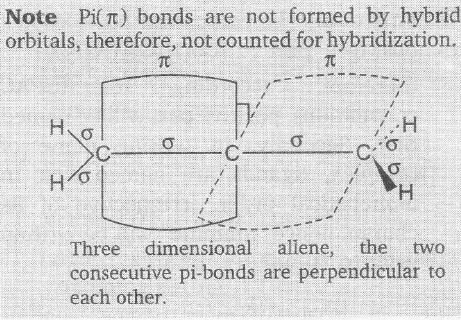
Allete is the name given to propdiene,
H2C=C=CH2
The hybridization of an atom is determined by determining the number of hybrids orbitals ar that atom which is equal to the number of sigma (σ) bonds plus a number of lone pairs at the concerned atom.
Here, the terminal carbons have only three sigma bonds associated with them, therefore, hybridization of terminal carbons is sp3. The central carbon has only two sigma bonds associated, hence hybridization at central carbon is sp