Answer:
Option C
Explanation:
A monosubstituted benzoic acid is stronger than a monosubstituted phenol as the former is a carboxylic acid. Among the given substituted benzoic acid, ortho-hydroxy acid is the strongest acid although -OH causes electron donation by resonance effect which tends to decrease acid strength. It is due to a very high stabilization of conjugate base by intramolecular H-bond which outweight the electron-donating resonance effect of _OH.
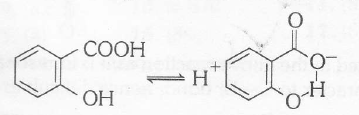
The overall order of acid-strength of given four acids is ortho-hydro xy benzoic acid
$(pK_{a}=2.98)>$ Toluic acid $pK_{a}=4.37) >$ p-hydroxybenzoic acid $(pK_{a}=4.58)>$
p-nitrophenol $(pK_{a}=7.15)$