Answer:
Option B
Explanation:
$Ni^{2+}+4CN^{-} \rightarrow [Ni(CN)_{4}]^{2-}$ . Here $Ni^{2+}$ has d8 configuration with CN- as strong ligand.
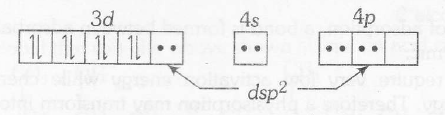
$d^{8}$ configuration in strong ligand field gives dsp2-hybridisation , hence square planar geometry
$Ni^{2+} +4Cl^{-} \rightarrow [NiCl_{4}]^{2-}$
Here $Ni^{2+}$ has $d^{8}$ -configuration with $Cl^{-}$ as weak ligand
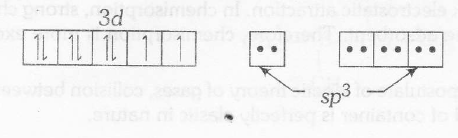
d8-configuration in weak ligand field gives sp3 hybridisation, hence tetrahedral geometry Ni2+ with H2O forms
$[Ni(H_{2}O)_{6}]^{2+}$ complex and H2O is a weak ligand
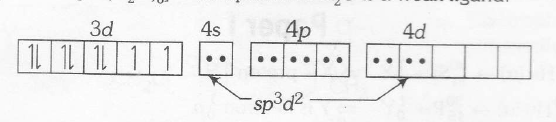
Therefore , $Ni(H_{2}O)^{2+}$ has octahedral geometry