Answer:
Option A
Explanation:
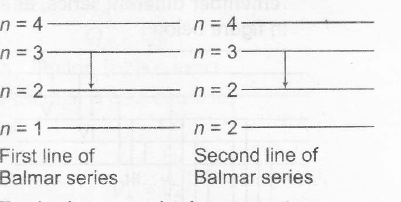
For hydrogen or hydrogen type atoms
1λ=RZ2(1n2f−1n2i)
In the transition from ni→nf
∴ λ∝1Z2(1n2f−1n2i)
∴ λ2λ1=Z21(1n2f−1n2i)1Z22(1n2f−1n2i)2
λ2=λ1Z21(1n2f−1n2i)1Z22(1n2f−1n2i)2
Substituting the values , we have
Å(6561Å)(1)2(122−132)(2)2(122−142)=1215Å
∴ correct option is (a)
Analysis of Question
(i) Question is simple.
(ii) In modern physics, mostly questions are asked on the emission of a photon by the transition of electrons from some higher energy state to some lower energy state
(iii) For hydrogen and hydrogen-like atoms which have onlY single
electron, we can use the formula
1λ=RZ2(1n2f−1n2i)
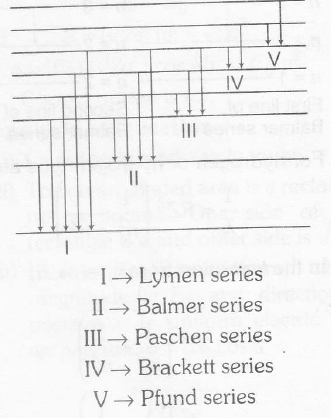
(iv) Further, the student should also remember different series, as shown
in figure below