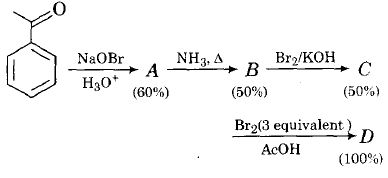In the following reactions sequence, the amount of D (in gram) formed from 10 moles of acetophenone is ...........
(Atomic weights in g mol -1 : H=1, C=12, N=14, O=16, Br= 80. The yield (%) corresponding to the product in each step is given in the parenthesis)