Answer:
Option A
Explanation:
Decay scheme is,
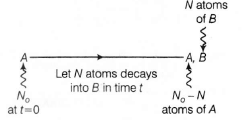
Given $\frac{N_{B}}{N_{A}}=0.3=\frac{3}{10}$
$\Rightarrow\frac{N_{B}}{N_{A}}=\frac{30}{100}$
So, N0 =100+30=130 atoms
By using $N= N_{0}e^{-\lambda t}$
We have , $100= 130e^{-\lambda t}$
$\Rightarrow \frac{1}{13}=e^{-\lambda t}\Rightarrow log1 .3=\lambda t$
If T is half-life, then $t=\frac{\log_{e}{2}}{T}$
$\Rightarrow \log_{}{1.3}=\frac{\log_{e}{2}}{T}.t$
$\therefore t=\frac{T.\log_{}{(1.3)}}{\log_{e}{2}}$