Answer:
Option B,D
Explanation:
$P_{4}O_{10}$ is a dehydrating agent and converts $HNO_{3}$ into $N_{2}O_{5}$
$2HNO_{3}\rightarrow N_{2}O_{5}+H_{2}O$
$P_{4}O_{10}+6H_{2}O\rightarrow 4H_{3}PO_{4}$
(a) $P_{4}+20HNO_{3}\rightarrow4H_{3}PO_{4}+20NO_{2}+4H_{2}O$
Thus , (a) is incorrect.
(b) $N_{2}O_{5}$ has no unpaired electron and is thus, diamagnetic , (b) is correct
(c) 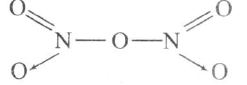
There is no N-N bond, thus (c) is incorrect
(d) $N_{2}O_{5}+Na\rightarrow NaNO_{3}+NO_{2}$
$N_{2}O_{5}$ vapours of brownish colour. Thus, (d) is correct.