Answer:
Option C
Explanation:
In the first process $p_{i}V_{i}^{\gamma}=p_{f}V_{f}^{\gamma}$
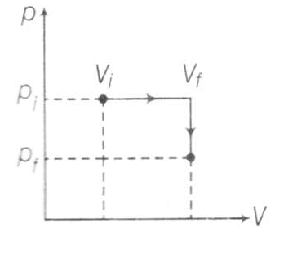
$\Rightarrow \frac{p_{i}}{p_{f}}=(\frac{V_{f}}{V_{i}})^{\gamma}\Rightarrow32=8^{\gamma}$
$\gamma =\frac{5}{3}$ ......(i)
For the two step process
$W= P_{i}(V_{f}-V_{i})=10^{5}(7\times 10^{-3})$
$W= 7\times 10^{2}$ J
$\triangle U= \frac{f}{2}(p_{f}V_{f}-p_{i}V_{i})$
$=\frac{I}{\gamma-1}(\frac{1}{4}\times 10^{2}-10^{2})$
$\triangle U= -\frac{3}{2}.\frac{3}{4}\times 10^{2}=-\frac{9}{8}\times 10^{2}J$
$Q-W=\triangle U$
$\Rightarrow 7\times 10^{2}-\frac{9}{8}\times 10^{2}$
$=\frac{47}{8}\times 10^{2}J =588J$